The Trump administration announced a major change on Tuesday to U.S. COVID-19 vaccination policy: healthy children and younger adults will no longer be recommended to get an annual COVID-19 vaccine.
While some celebrated the changes in recommendations, Make America Healthy Again (MAHA) influencer Diana Atieh was disappointed with the decision, as the vaccine is still recommended for other groups.
“It’s really unfortunate to see the Trump administration and FDA approve and push the COVID vaccine, especially to those who already have preexisting health conditions,” Atieh told Newsweek on Tuesday.
Why It Matters
This policy marks a dramatic reversal from previous federal guidance that encouraged annual COVID-19 vaccinations for nearly all age groups in the United States. The shift is expected to impact eligibility for more than 100 million Americans, with routine access now restricted to groups at elevated risk for severe illness.
Experts warned the decision could complicate access to vaccines for healthy individuals, leaving many uncertain whether they qualify or if insurance will cover the cost. The move comes as part of broader changes implemented by officials critical of sweeping vaccine mandates and as new leadership oversees national health agencies.
Michael Ciaglo/Getty Images
What To Know
The decision, authored by FDA Commissioner Marty Makary and vaccine chief Vinay Prasad, focuses on vaccine eligibility for adults 65 and older.
In addition to Americans over six months old who have at least one serious health condition, including asthma, diabetes, cancer or obesity, pregnant women are also listed among those encouraged to get vaccinated under the new guidelines.
Younger, healthy adults and children are not recommended to get vaccinated each year for COVID-19.
“The COVID vaccine was shown to not help with outcomes of those that contracted COVID-19, especially when looking at hospitalizations and deaths,” Atieh, who goes by @diaryofacrunchymom on Instagram, told Newsweek.
She sent the following image to defend her claims:
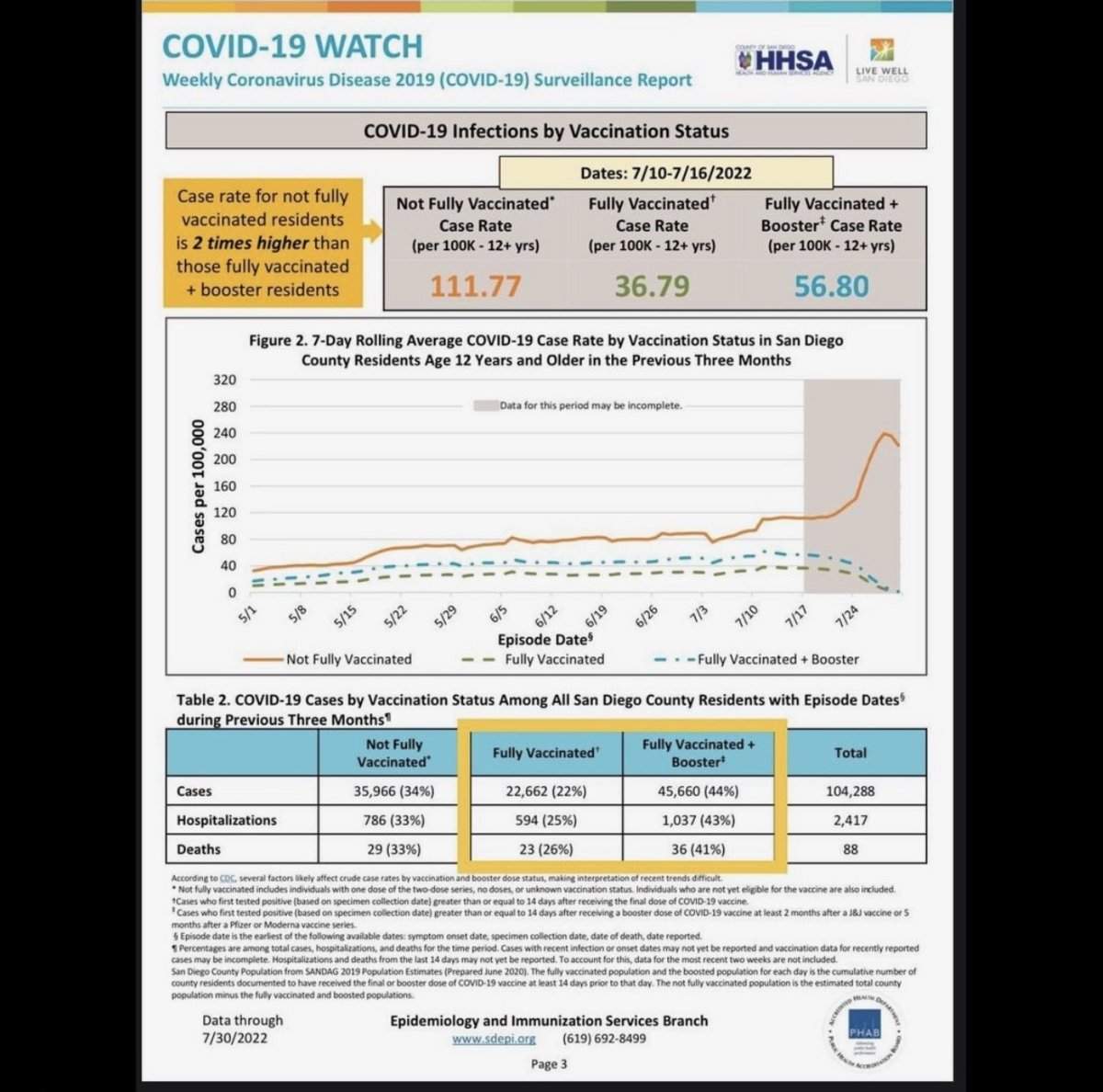
SDEPI
In addition to “the push of the COVID vaccine on pregnant women” being concerning, Atieh said that “we are unable to examine long-term effects on babies whose mothers have received mRNA vaccines while in utero.”
“While I still have high hopes of [Health and Human Services Secretary Robert F. Kennedy, Jr.] stepping in and changing the system to highlight risks associated with vaccines, here is a prime example of just how influential Big Pharma still is,” she said.
“It’s very important for the FDA to also show the risks with any medical procedure like vaccinations, and here they have not done that—which shows how little they care about informed consent for consumers.”
FDA leaders estimated that more than 100 million Americans would still qualify for COVID vaccines under the new rules. However, younger and otherwise healthy individuals cannot easily access the updated shots unless new Centers for Disease Control and Prevention recommendations or insurance policies expand coverage. Eligibility could ultimately depend on individual health providers and insurance companies.
Seventy-four percent of American adults have at least one condition increasing their risk for severe COVID-19, according to CDC data. Despite this, the policy’s narrower focus means that routine shots for otherwise healthy people under 65 may no longer be covered.
Makary and Prasad argued that the U.S. had previously taken an “aggressive” approach compared to Europe and questioned whether additional boosters were justified for healthy individuals with prior infection or vaccination.
What People Are Saying
Georgia Representative Marjorie Taylor Greene told Newsweek: “I’m thrilled with the FDA’s new guidelines for COVID vaccines! No one should have ever been forced to take an unproven and experimental shot. It turned out deadly with miscarriages and myocarditis going through the roof after the COVID shot was forced upon the American people.
“Thank you to Secretary Kennedy for rightly updating the FDA’s guidance. Big Pharma has made enough money off the government’s recommendations. It’s time for the American people to have health freedom again.”
Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia and vaccine expert, told The Washington Post: “Is the pharmacist going to determine if you’re in a high-risk group? The only thing that can come of this will make vaccines less insurable and less available.”
FDA officials wrote in The New England Journal of Medicine earlier this month: “We simply don’t know whether a healthy 52-year-old woman with a normal BMI who has had COVID-19 three times and has received six previous doses…will benefit from the seventh dose.”
What Happens Next
The CDC’s advisory committee is scheduled to meet on June 25 to finalize vaccination group recommendations for the fall COVID-19 campaign, including possible clarification for pregnant women and others not captured in the FDA’s risk definitions.
