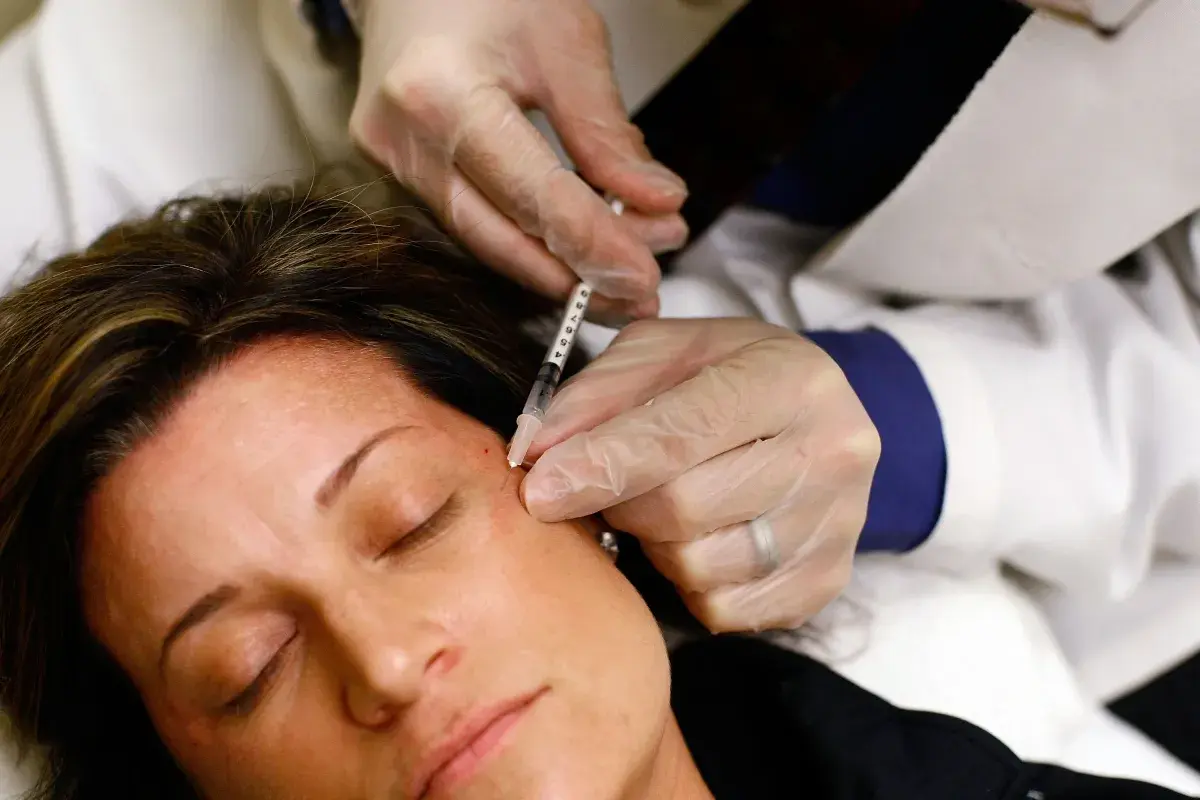
The U.S. Food and Drug Administration (FDA) is warning companies to stop marketing unapproved Botox products after reports of a rare but serious illness linked to botulism.
Why It Matters
Botulinum toxin, commonly called Botox, is used to temporarily reduce wrinkles by paralyzing facial muscles. While it is considered safe, unapproved injections or counterfeit injections can come with serious side effects, including botulism, which can be fatal.
What To Know
On Wednesday, the FDA sent warning letters to 18 websites accused of illegally selling unapproved and misbranded Botox products. FDA officials reported injuries and toxic side effects associated with these products, noting the ongoing risk of botulism, a rare condition resulting when the toxin spreads beyond the injection site, causing symptoms such as muscle weakness, difficulty swallowing or breathing, and, in severe cases, death.
The FDA’s enforcement targeted online sellers marketing counterfeit or unauthorized versions that had not been verified by the agency. The websites the FDA has warned about are:
- aesthetic-essentials.com
The warning follows an uptick in adverse event reports. The Centers for Disease Control and Prevention (CDC) documented incidents involving at least 22 individuals from 11 states between November 2023 and March 2024 after receiving suspicious injections mainly for cosmetic purposes, often administered outside licensed health care settings, including homes and spas.
Most affected individuals were women aged 25 to 59. Symptoms ranged from drooping eyelids to respiratory distress, and 11 required hospitalization.
FDA-approved Botox should be administered by licensed, trained professionals.
Consumers are urged to verify both the product and the provider before receiving any botulinum toxin injection. If symptoms emerge after treatment—such as vision changes, muscle weakness, or breathing issues—patients should seek immediate medical attention.
What People Are Saying
FDA Commissioner Marty Makary said in a statement: “Unapproved and misbranded Botox products carry serious health risks. Today we’re taking action to protect American consumers and prevent online entities from selling these dangerous products.”
Oma Agbai, board-certified dermatologist, said in a statement through the University of California, Davis: “When considering cosmetic procedures, patients should take an active role in advocating for their own safety.”
What Happens Next
The FDA, CDC, and state health departments continue to monitor adverse event reports linked to unauthorized botulinum toxin products. The FDA also encouraged anyone who has received an injection to seek immediate medical care if they have symptoms of botulism, which include trouble swallowing or breathing.
